-
 E-mail
guhaixin0724@gmail.com
E-mail
guhaixin0724@gmail.com
-
 Call Us
008613683147042
Call Us
008613683147042
 E-mail
guhaixin0724@gmail.com
E-mail
guhaixin0724@gmail.com
 Call Us
008613683147042
Call Us
008613683147042
2025-04-23 Views: 480
Warm Tip: If you want to know more information, like quotation, products, solutions, etc., please Click here ,and contact us online.

Gold(I) sulfide (Au₂S) is essentially insoluble in water and exhibits strong resistance to oxidation under ambient conditions, necessitating aggressive pretreatment to liberate gold.
Gold grains in sulfide ores are typically sub-microscopic—ranging from 70 μm to 2 mm—and occur encapsulated within pyrite (FeS₂) or arsenopyrite (FeAsS), impeding direct leaching.
These ores form in hard-rock settings such as quartz-sulfide veins, black shale horizons, and polymetallic sulfide deposits, where hydrothermal fluids precipitate gold alongside sulfide minerals.
They frequently co-occur with silver, copper, lead, zinc, and organic carbon, factors that further complicate downstream extraction.
Refractory behavior arises because ultra-fine gold is occluded within sulfide matrices, leading to direct cyanidation recoveries often below 60% without pretreatment.
Arsenic-bearing minerals and carbonaceous “preg-robbers” sequester dissolved gold-cyanide complexes, exacerbating reagent consumption and reducing recovery.
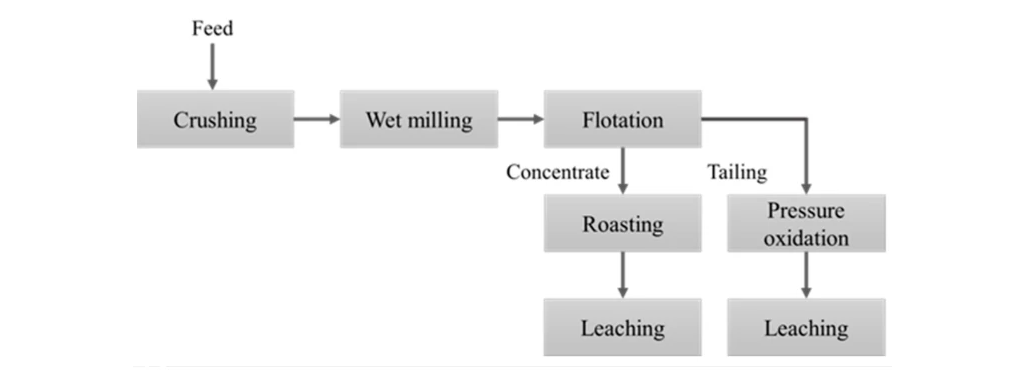
2.1.1 Roasting (650–800 °C)
Roasting oxidizes sulfides to oxides—e.g., 4 FeS₂ + 11 O₂ → 2 Fe₂O₃ + 8 SO₂—disrupting encapsulation and freeing gold for leaching.
Despite its high efficacy for sulfide-rich concentrates, roasting is energy-intensive and generates substantial SO₂ emissions that require scrubbing.
2.1.2 Bio-Oxidation (BIOX)
BIOX employs acidophilic bacteria (e.g., Acidithiobacillus spp.) to oxidize sulfide minerals at ambient pressure, offering a greener alternative but requiring 2–3 weeks for complete mineral breakdown.
2.1.3 Pressure Acid Oxidation (POX)
In POX, sulfide slurries are treated in high-pressure autoclaves with H₂SO₄ and O₂, converting sulfides to sulfates and scorodite (FeAsO₄·2H₂O), which can be safely disposed.
This method typically boosts gold recovery by at least 10% over roasting and immobilizes arsenic in solid form.
2.2.1 Smelting
Smelting of sulfide concentrates with fluxes (e.g., borax) separates molten slag from precious metal-rich bullion, especially suited to high-grade concentrates.
2.2.2 Selective Sulfidation
Selective sulfidation transforms gold compounds into sulfide phases, enhancing their affinity for subsequent flotation separation.
2.3.1 Cyanidation
Alkaline NaCN leaching (4 Au + 8 CN⁻ + O₂ → 4 [Au(CN)₂]⁻) remains the industry standard, often preceded by oxidation pretreatment to achieve recoveries up to 89% in combined flotation-roast-cyanidation circuits.
2.3.2 Non-Cyanide Leaching
Thiosulfate: Alkaline thiosulfate with Cu–NH₃ catalysts offers low toxicity but demands precise pH control.
Halides: Brine systems (NaBr/FeCl₃) can recover ~75% of gold in 6 h, though reagent costs and corrosivity pose challenges.
Thiourea/Polysulfides: Fast kinetics with high dissolution rates but elevated reagent costs and stability issues.
2.4.1 Selective Flotation
Selective collectors (e.g., xanthates, dithiophosphates) concentrate gold-bearing sulfides with recoveries exceeding 90% through pyrite or sphalerite flotation.
2.4.2 Gravity-Flotation Hybrid
Centrifugal gravity concentrators (e.g., Knelson) recover coarse gold, while flotation targets fine sulfide-bound gold, achieving combined recoveries around 85%.
2.5.1 Nanoparticle Catalysts
Lab-scale studies using nano-TiO₂ or noble-metal nanoparticles demonstrate up to 20% faster gold leaching from refractory matrices.
2.5.2 Ionic Liquids
Ionic solvents (e.g., [Bmim][HSO₄]) show promise for selective gold dissolution under mild conditions, though industrial viability remains under investigation.
Tailings management must stabilize arsenic and heavy metals—commonly via lime addition or bio-solidification—to prevent leaching into groundwater.
Roasting is significantly more energy-intensive than bio-oxidation, often consuming double the energy per tonne processed, thereby increasing greenhouse gas footprints.
An Australian operation integrating POX with downstream cyanidation achieved gold recoveries exceeding 90% while immobilizing arsenic as scorodite in autoclave solids.
At the Shuiyindong gold mine (Guizhou Province), acid POX pretreatment followed by ammonium thiosulfate leaching improved laboratory gold recovery from 10% to 79% and demonstrated potential to cut cyanide use by 40%.
Effective extraction of refractory sulfide gold ores hinges on tailored pretreatments—roasting, BIOX, or POX—to liberate occluded gold, followed by cyanidation or eco-friendly lixiviants. Hybrid flotation and gravity circuits enhance recovery for coarse and fine fractions. Addressing environmental and energy concerns through closed-loop systems and emerging technologies (nanocatalysts, ionic liquids) will be pivotal for sustainable, high-efficiency gold production.
If you have any questions or needs, please feel free to contact us!
No. 188, Xinhai Street, high-tech Industrial Park, Fushan District, Yantai, Shandong, China.

Please leave your message here! We will send detail technical info and quotation to you!